In a clinical study of adults with newly diagnosed Ph+ CML-CP
SCEMBLIX had better response rates than commonly used TKIs*
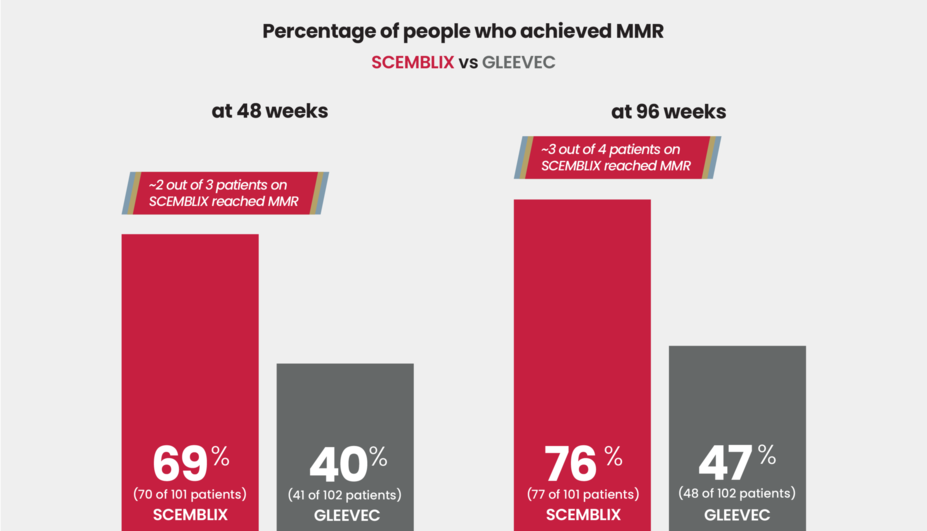
More people reached major molecular response (MMR) at 48 and 96 weeks
For adult patients with newly diagnosed Ph+ CML in chronic phase, SCEMBLIX is the only medication that was compared against all commonly used TKIs.
Reaching major molecular response (MMR) is an important treatment milestone that measures the amount of leukemic cells in your blood by checking the abnormal BCR::ABL1 levels. MMR is reached when the abnormal gene level in your blood has decreased by at least 1,000 times compared to the level measured at diagnosis.
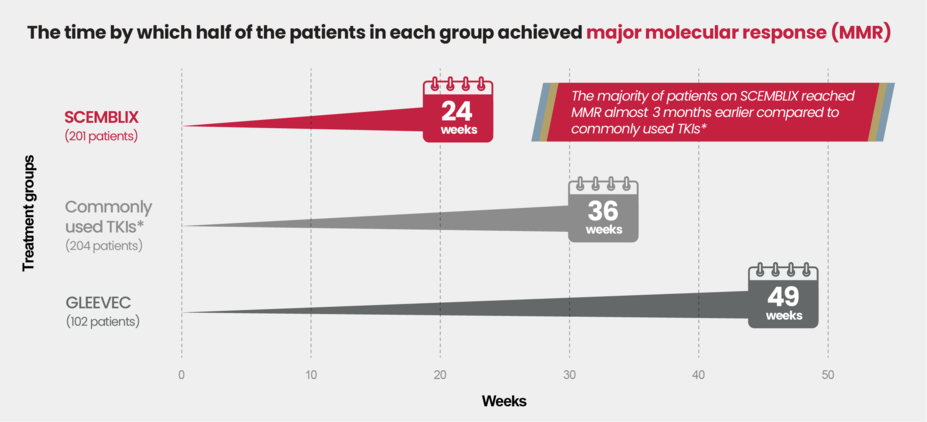
SCEMBLIX worked fast
If you're an adult with newly diagnosed Ph+ CML in chronic phase, ask your health care team if SCEMBLIX may be the right treatment to start on.
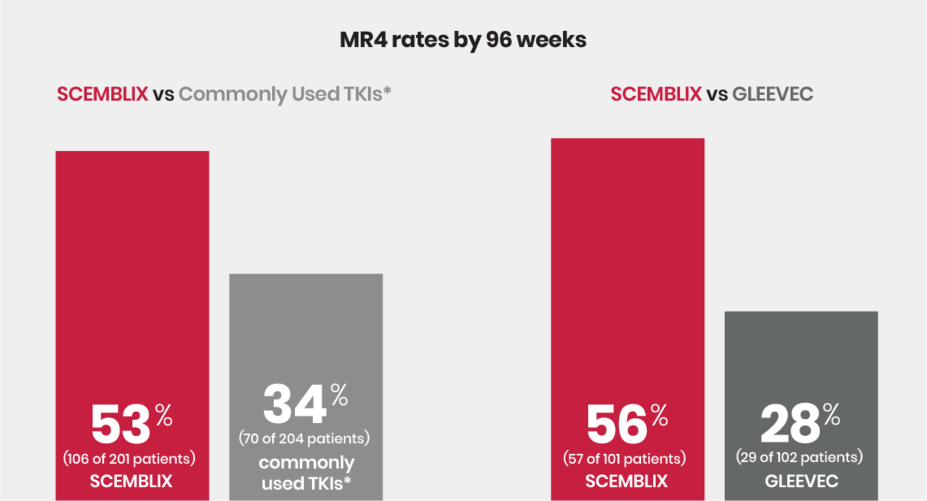
MR4 rates for SCEMBLIX and commonly used TKIs*
MR4 is the next milestone where the response is 10 times larger than MMR. MR4 is reached when the abnormal gene level in the blood has decreased by at least 10,000 times (4-log reduction) compared to the level measured at diagnosis. MR4 is the next milestone after MMR.
MR4 rates by 48 weeks:
41% (82 of 201 patients) on SCEMBLIX and 22% (45 of 204 patients) on commonly used TKIs*
46% (46 of 101 patients) on SCEMBLIX and 16 (16 of 102 patients) on GLEEVEC
Compared to commonly used TKIs,* more people were able to stay on SCEMBLIX by 96 weeks
With a minimum of 96 weeks of follow-up, 82% of people on SCEMBLIX were still on treatment compared to 60% of people on the most commonly used TKIs.*
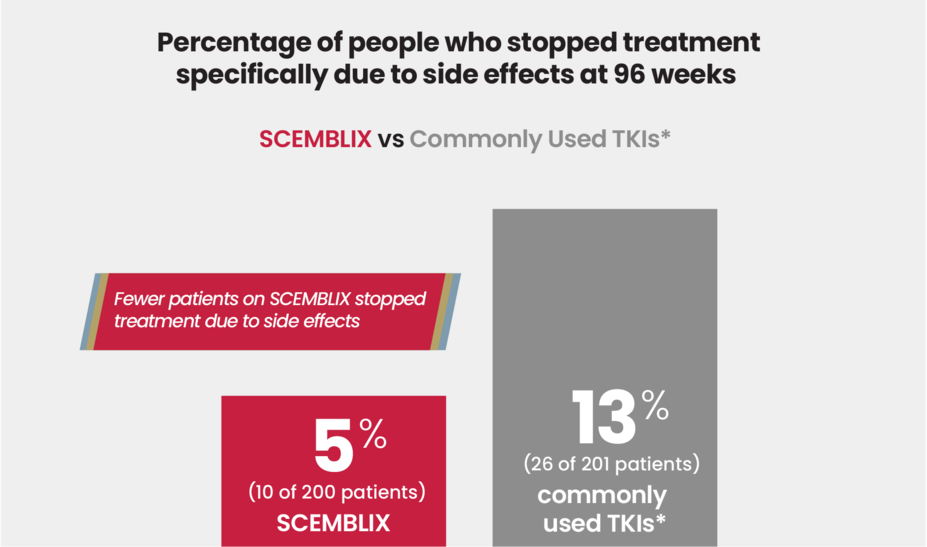
Common side effects
In this clinical study, the most common side effects reported (≥ 20%) with SCEMBLIX were pain in the muscles, bones, or joints, and rash.
These are not all of the possible side effects of SCEMBLIX.
Review the serious and most common side effects of SCEMBLIX. Learn more
Ask your health care team about SCEMBLIX as your first treatment |
Ph+ CML, Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase
*SCEMBLIX was studied vs GLEEVEC® (imatinib), TASIGNA® (nilotinib), Sprycel ® (dasatinib), and Bosulif ® (bosutinib). Sprycel is a registered trademark of Bristol-Myers Squibb Company. Bosulif is a registered trademark of Pfizer Inc.
†Limitations apply. Valid only for those with private insurance. The Program includes the Co-Pay Plus offer, Plus Card (if applicable), and Rebate, with a combined annual limit. Patient is responsible for any costs once limit is reached in a calendar year. Program not valid (i) under Medicare, Medicaid, TRICARE, VA, DoD, or any other federal or state healthcare program, (ii) where patient is not using insurance coverage at all, (iii) where the patient’s insurance plan reimburses for the entire cost of the drug, or (iv) where product is not covered by patient’s insurance. The value of this program is exclusively for the benefit of patients and is intended to be credited towards patient out-of-pocket obligations and maximums, including applicable co-payments, coinsurance, and deductibles. Program is not valid where prohibited by law. Patient may not seek reimbursement for the value received from this program from other parties, including any health insurance program or plan, flexible spending account, or healthcare savings account. Patient is responsible for complying with any applicable limitations and requirements of their health plan related to the use of the Program. Valid only in the United States and Puerto Rico. This Program is not health insurance. Program may not be combined with any third-party rebate, coupon, or offer. Proof of purchase may be required. Novartis reserves the right to rescind, revoke, or amend the Program and discontinue support at any time without notice.